An influential US government advisory panel has rejected plans to give COVID-19 booster jabs to most Americans - only backing them for people aged 65 and over, and those at high risk of severe disease.
The decisions were made by a committee of experts who advise the Food and Drug Administration (FDA) and deliver a huge blow to the Biden administration.
US President Joe Biden announced a plan, around a month ago, to offer booster jabs of both Pfizer and Moderna vaccines to nearly all Americans eight months after they get their second dose.
But in a surprising turn, the panel overwhelmingly rejected boosters for everyone, by a vote of 16-2.
Experts said there was a lack of safety data surrounding extra COVID vaccine doses and raised doubts about the value of mass boosters, rather than ones targeted to specific groups.
Dr Cody Meissner of Tufts University said: "I don't think a booster dose is going to significantly contribute to controlling the pandemic and I think it's important that the main message we transmit is that we've got to get everyone two doses."
In a second vote, the group endorsed the extra jab for people aged 65 and over and those who run a high risk of severe disease if they contract the virus.
However, there is still hope for the US government as the offering of boosters is also subject to approval by the Centers for Disease Control and Prevention (CDC).
It has said it is considering boosters for older people, nursing home residents and front line health care workers, rather than all adults.
Dr Amanda Cohn of the CDC said: "At this moment it is clear that the unvaccinated are driving transmission in the United States."
A CDC advisory panel is due to discuss the issue on Wednesday.
Separate decisions will need to be taken by both the FDA and the CDC in order for people who received Moderna or Johnson & Johnson vaccines to get boosters.
Joe Biden's top health advisers, including the heads of the FDA and CDC, first announced plans for widespread booster shots in mid-August.
They labelled the week starting 20 September as an all-but-certain start date but that was before FDA staff scientists had completed their own assessments of the data.
Scientists have been divided on the issue of extra vaccine doses, with the World Health Organisation strongly objecting to rich nations giving them out while poor countries still don't have enough to offer a first jab to everyone.
The US government has argued that the plan is not an us-or-them choice, noting that the country is supplying large quantities of vaccines to the rest of the world.
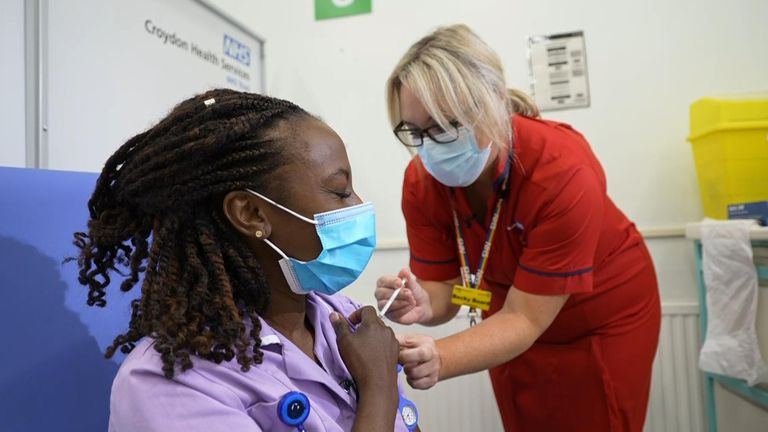
In the UK, the NHS has already started issuing COVID booster jabs - as figures show that more than one in five adults under 40 have still not had any jab at all.
Over 50s, people in care homes, frontline health and social care workers and vulnerable people between 16 and 49 are among those who will be offered a third dose.
Hospital hubs started giving third doses to health and social care workers on Thursday, NHS England said, with other eligible people now being identified.
GP vaccination services will follow in the coming days and the full rollout will start next week as more vaccination centres and pharmacy centres finish final checks.
At least six months must have passed since the person's second dose before they can be offered a booster.
NHS England said people would be contacted by their GP or the National Booking Service when they become eligible.
https://news.google.com/__i/rss/rd/articles/CBMilwFodHRwczovL25ld3Muc2t5LmNvbS9zdG9yeS9jb3ZpZC0xOS1wYW5lbC1yZWplY3RzLWJvb3N0ZXItamFicy1wbGFuLWZvci1hbGwtYW1lcmljYW5zLXdpdGgtZ3JlZW4tbGlnaHQtZ2l2ZW4tdG8tb3Zlci02NXMtYW5kLXZ1bG5lcmFibGUtZ3JvdXBzLTEyNDEwODY10gGbAWh0dHBzOi8vbmV3cy5za3kuY29tL3N0b3J5L2FtcC9jb3ZpZC0xOS1wYW5lbC1yZWplY3RzLWJvb3N0ZXItamFicy1wbGFuLWZvci1hbGwtYW1lcmljYW5zLXdpdGgtZ3JlZW4tbGlnaHQtZ2l2ZW4tdG8tb3Zlci02NXMtYW5kLXZ1bG5lcmFibGUtZ3JvdXBzLTEyNDEwODY1?oc=5
2021-09-17 23:16:53Z
52781879721858

Tidak ada komentar:
Posting Komentar